The First Law of Thermodynamics: Control Volumes
advertisement
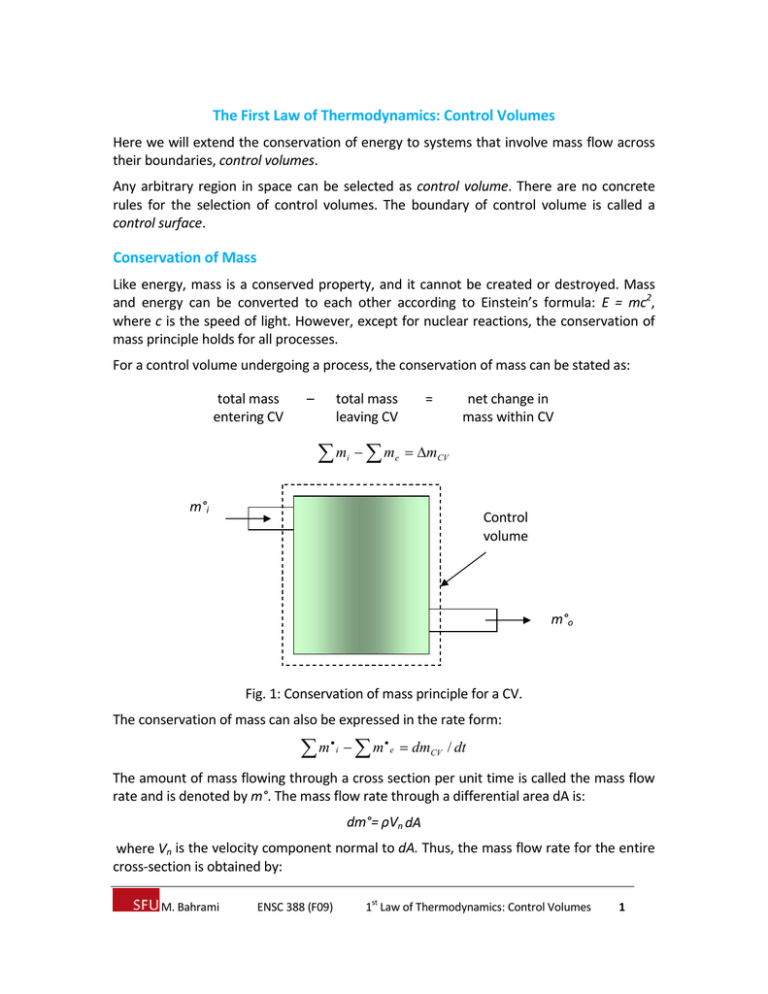
The First Law of Thermodynamics: Control Volumes Here we will extend the conservation of energy to systems that involve mass flow across their boundaries, control volumes. Any arbitrary region in space can be selected as control volume. There are no concrete rules for the selection of control volumes. The boundary of control volume is called a control surface. Conservation of Mass Like energy, mass is a conserved property, and it cannot be created or destroyed. Mass and energy can be converted to each other according to Einstein’s formula: E = mc2, where c is the speed of light. However, except for nuclear reactions, the conservation of mass principle holds for all processes. For a control volume undergoing a process, the conservation of mass can be stated as: total mass entering CV – total mass leaving CV m m i e = net change in mass within CV mCV m°i Control volume m°o Fig. 1: Conservation of mass principle for a CV. The conservation of mass can also be expressed in the rate form: m i m e dmCV / dt The amount of mass flowing through a cross section per unit time is called the mass flow rate and is denoted by m°. The mass flow rate through a differential area dA is: dm°= ρVn dA where Vn is the velocity component normal to dA. Thus, the mass flow rate for the entire cross‐section is obtained by: st M. Bahrami ENSC 388 (F09) 1 Law of Thermodynamics: Control Volumes 1 m Vn dA (kg/s) A Assuming one‐dimensional flow, a uniform (averaged or bulk) velocity can be defined: m°= ρ V A (kg/s) where V (m/s) is the fluid velocity normal to the cross sectional area. The volume of the fluid flowing through a cross‐section per unit time is called the volumetric flow, V°: V Vn dA VA (m 3 /s) A The mass and volume flow rate are related by: m°=ρV°= V°/ v. Conservation of Energy For control volumes, an additional mechanism can change the energy of a system: mass flow in and out of the control volume. Therefore, the conservation of energy for a control volume undergoing a process can be expressed as total energy crossing boundary as heat and work + total energy of – mass entering CV total energy of mass leaving CV = net change in energy of CV Q W Ein ,mass Eout ,mass ECV This equation is applicable to any control volume undergoing any process. This equation can also be expressed in rate form: Q W dEin ,mass / dt dEout ,mass / dt dECV / dt Mass in Control volume Q W Mass out Fig. 2: Energy content of CV can be changed by mass flow in/out and heat and work interactions. Work flow: is the energy that is required to push fluid into or out of a control volume. Consider an imaginary piston (that pushes the fluid to CV) where the fluid pressure is P and the cross sectional area is A. The force acting on the piston is F = PA. st M. Bahrami ENSC 388 (F09) 1 Law of Thermodynamics: Control Volumes 2 A Flow direction P P s Fig. 3: schematic for flow work. The work done in pushing the fluid is: Wflow = F.s = PA.s = PV (kJ) or in a unit basis, wflow = Wflow / m = Pv (kJ/kg) Note that the flow work is expressed in terms of properties. The flow work can also be written as a rate equation. The fluid entering or leaving a control volume possesses an additional form of energy (flow energy Pv). Therefore, the total energy of a flowing fluid on a unit‐mass basis (denoted by θ) becomes: θ = Pv + e = Pv + (u + ke + pe) (kJ/kg) Recall that enthalpy is defined as: h = u + Pv. Therefore, the above equation becomes: θ = h + ke + pe = h + V2 / 2 + gz (kJ/kg) The property θ is called methalpy. By using enthalpy instead of internal energy, the energy associated with flow work into/out of control volume is automatically taken care of. This is the main reason that enthalpy is defined! Steady‐State Flow Process A process during which a fluid flows through a control volume steadily is called steady‐ state process. A large number of devices such as turbines, compressors, and nozzles operates under the same conditions for a long time and can be modeled (classified) as steady‐flow devices. The term steady implies no change with time. The term uniform implies no change with location over a specified region. A steady flow is characterized by the following: st M. Bahrami ENSC 388 (F09) 1 Law of Thermodynamics: Control Volumes 3 1‐ No properties within the CV change with time. Thus, volume, mass, and energy of CV remains constant. As a result, the boundary work is zero. Also, total mass entering the CV must be equal to total mass leaving CV. 2‐ No properties change at the boundary of the CV with time. It means that the mass flow rate and the properties of the fluid at an opening must remain constant during a steady flow. 3‐ The heat and mass interactions between the CV and its surroundings do not change with time. Using the above observation, the conservation of energy principle for a general steady‐ flow system with multiple inlets and exits can be written as: 2 2 Ve Vi Q W m he gz i gz e mi hi 2 2 Q W m e e mi i e Nozzles and Diffusers A nozzle is a device that increases the velocity of a fluid at the expense of pressure. A diffuser is a device that increases the pressure of a fluid by slowing it down. The cross sectional area of a nozzle decreases in the flow direction for subsonic flows and increase for supersonic flows. W° = 0 A1 > A2 1 2 Nozzle V2 > V1 P1 < P2 Control Volume Q° = 0 Fig. 4: Schematic of nozzle. For a nozzle, common assumptions and idealizations are: Q° = 0, no time for heat transfer, due to high velocity W° = 0, nozzles include no shaft or electric resistance wires ΔPE = 0, no change in fluid elevation. Mass equation for nozzles becomes: m°1 = m°2 or ρ1A1V1 = ρ2A2V2 st M. Bahrami ENSC 388 (F09) 1 Law of Thermodynamics: Control Volumes 4 Energy balance: Q° ‐ W° = m°1 θ1 ‐ m°2 θ2 (h + V2 / 2)at inlet = (h + V2 / 2)at exit Diffusers are exactly the same device as nozzles; the only difference is the direction of the flow. Thus, all equations derived for nozzles hold for diffusers. W° = 0 A1 < A2 2 1 Diffuser V1 > V2 P1 < P2 Control Volume Q° = 0 Fig. 5: Schematic for diffuser. Example 1: Nozzle Steam enters a converging‐diverging nozzle operating at steady state with P1 = 0.05 MPa, T1 = 400 °C and a velocity of 10 m/s. The steam flows through the nozzle with negligible heat transfer and no significant change in potential energy. At the exit, P2 = 0.01 MPa, and the velocity is 665 m/s. The mass flow rate is 2 kg/s. Determine the exit area of the nozzle, in m2. Assumptions: 1. Steady state operation of the nozzle 2. Work and heat transfer are negligible, Q° = W°= 0. 3. Change in potential energy from inlet to exit is negligible, ΔPE = 0. The exit area can be calculated from the mass flow rate m°: A2 = m°v2 / V2 We need the specific volume at state 2. So, state 2 must be found. The pressure at the exit is given; to fix the state 2 another property is required. From the energy equation enthalpy can be found: V22 V12 h2 h1 2 2 h2 h1 1 2 V1 V22 2 From Table A‐6, h1 = 3278.9 kJ/kg and velocities are given. st M. Bahrami ENSC 388 (F09) 1 Law of Thermodynamics: Control Volumes 5 10 2 665 2 m 1 N 1 kJ h2 3278.9 kJ / kg 2 3 3057.84 kJ / kg 2 s 1 kg.m / s 10 N .m 2 From Table A‐6, with P2 = 0.01 MPa, and h2 = 3057.84 kJ/kg, (using interpolation) one finds T2 = 300 °C (approximately) and v2 = 26.445 m3/kg. The exit area is: A2 2 kg / s 26.445 m 3 / kg 0.07955 m 2 665 m / s Fig. 6: Converging‐diverging nozzle. Turbines and Compressors In steam, gas, or hydroelectric power plants, the device that derives the electric generator is turbine. The work of turbine is positive since it is done by the fluid. Compressors, pumps, and fans are devices used to increase the pressure of the fluid. Work is supplied to these devices, thus the work term is negative. Common assumptions for turbines and compressors: Q° = 0. ΔPE = ΔKE = 0. Example 2: Turbine Steam enters a turbine at steady state with a mass flow rate of 4600 kg/h. The turbine develops a power output of 1000 kW. At the inlet the pressure is 0.05 MPa, the temperature is 400 °C, and the velocity is 10 m/s. At the exit, the pressure is 10 kPa, the quality is 0.9, and the velocity is 50 m/s. Calculate the rate of heat transfer between the turbine and surroundings, in kW. st M. Bahrami ENSC 388 (F09) 1 Law of Thermodynamics: Control Volumes 6 Assumptions: Steady‐state operation. The change in potential energy is negligible. The energy balance for the turbine is: CV Q CV W QCV WCV V22 V12 m h2 gz 2 m h1 gz1 2 2 1 m h2 h1 V22 V12 2 1 Control Volume Turbine Shaft W° Q° = 0 (negligible) 2 Fig. 7: Schematic for turbine. Using Table A‐6, h1 = 3278.9 kJ/kg, using Table A‐5 with x = 0.9, h2 = hf + x2 hfg = 191.83 kJ/kg + 0.9(2392.8 kJ/kg) = 2345.35 kJ/kg Therefore, h2 – h1 = 2345.35 – 3278.9 = ‐933.55 kJ/kg The specific kinetic energy change is: 1 2 V2 V12 0.5 50 2 10 2 2 ms 2 1N 1 kJ 1.2 kJ / kg 2 3 1 kg.m / s 10 N .m Q°CV = (1000 kW) + 4600 kg/h (‐831.8 + 1.2 kJ/kg) (1h / 3600 s) (1kW / 1 kJ/s) = ‐61.3 kW st M. Bahrami ENSC 388 (F09) 1 Law of Thermodynamics: Control Volumes 7 Note that change in specific kinetic energy is very small. Also Q°CV is small compared to W°CV. The negative sign in Q°CV means that heat transfer is from CV to surroundings. Throttling Valves Any kind of flow restricting devices that causes a significant pressure drop in the fluid is called throttling valve. Fig. 8: Three kinds of throttling valves. The pressure drop in fluid is often accompanied with a temperature drop in fluids. The magnitude of this temperature is governed by a property called the Joule‐Thomson coefficient. Common assumptions for throttling valves: Q° = 0 W° = 0 ΔPE = ΔKE = 0. The conservation of energy becomes: h2 = h1 u1 + P1v1 = u2 + P2v2 The flow energy increases during the process (P2v2> P1v1), and it is done at the expense of the internal energy. Thus, internal energy decreases, which is usually accompanied by a drop in temperature. st M. Bahrami ENSC 388 (F09) 1 Law of Thermodynamics: Control Volumes 8 For ideal gases h = h(T) and since h remains constant the temperature has to remain constant too. Mixture Chambers (Direct Contact Heat Exchangers) Mixture chambers are used to mix two streams of fluids. Based on mass principle, the sum of incoming flow equals to sum of leaving fluid. Control Volume Cold Mixing chamber Hot Water Water W°= 0 Q°= 0 Mixed water Fig. 9: T‐elbow is a mixing chamber. Common assumptions for mixing chamber: Q° = 0, W° = 0, ΔPE = ΔKE = 0. The conservation of energy becomes: Σ(m°h)inlet = Σ(m°h)outlet Heat Exchangers Heat exchangers are devices where two moving fluid streams exchange heat without mixing. Common assumptions for heat exchangers: W° = 0 ΔPE = ΔKE = 0. Q° could be different depending on the selected CV, see the next example. The mass and energy balance become: m1 m2 and m3 m4 m1 h1 m3 h3 m1 h2 m1 h4 st M. Bahrami ENSC 388 (F09) 1 Law of Thermodynamics: Control Volumes 9 1 Fluid A Heat 4 Fluid B Fluid B 3 Fluid A 2 Fig. 10: Schematic for heat exchanger. Example 3: Heat exchanger Engine oil is to be cooled by water in a condenser. The engine oil enters the condenser with a mass flow rate of 6 kg/min at 1 MPa and 70°C and leaves at 35°C. The cooling water enters at 300 kPa and 15°C and leaves at 25°C. Neglecting any pressure drops; determine a) the mass flow rate of the cooling water required, and b) the heat transfer rate from the engine oil to water. 1 Control Volume Water 15°C Heat 4 Oil 70°C 3 Oil 35°C 2 Water 25°C We choose the entire heat exchanger as our control volume, thus work transfer and heat transfer to the surroundings will be zero. From mass balance: m1 m2 mW and st M. Bahrami ENSC 388 (F09) 1 m3 m4 mOil Law of Thermodynamics: Control Volumes 10 The conservation of energy equation is: V2 V2 Q W me he e gze mi hi i gzi 2 2 me he me he mW h1 mOil h3 mW h2 mOil h4 mW h3 h4 mOil h2 h1 Assuming constant specific heat for both the oil and water at their average temperature, mW CP ,Oil T3 T4 mOil CP ,Water T2 T1 kJ 70 35 2.016 kg.C 6 kg / min 10.1 kg / min kJ 25 15 4.18 kg.C b) To determine the heat transfer from the oil to water, choose the following CV. The energy equation becomes: h mOil QOil C P ,Oil T4 T3 W mOil kJ 35 70 423.36 kJ / kg QOil 6 kg / min 2.016 kg.C Oil 35°C Control volume Oil 70°C 4 3 Q st M. Bahrami ENSC 388 (F09) 1 Law of Thermodynamics: Control Volumes 11